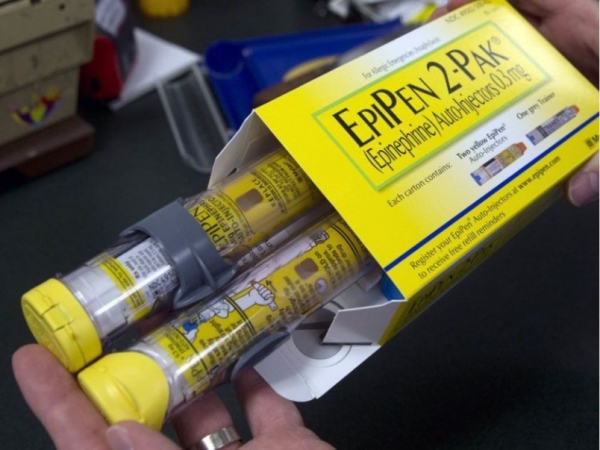
Earlier this year, Pfizer Canada, the pharmaceutical giant behind EpiPens, reported a shortage in both the 0.3 and 0.15-milligram versions of the drug. As the country’s only brand of life-saving epinephrine auto-injectors (EAIs), Pfizer’s announcement sent Health Canada scrambling for solutions as the shortage is predicted to persist into next year.
EAIs are used to deliver a fast dose of epinephrine—also called adrenaline—to someone experiencing a severe allergic reaction. This includes anaphylaxis, the most severe allergic reaction, which can ultimately lead to death. According to a report by the Canadian Institute for Health Information, allergic reactions make up one per cent of all emergency room visits every year.
Pfizer Canada issued a statement in April, attributing the shortage to “delays at the manufacturing facility and limited third-party quantities of a component for the product.”
When The McGill Tribune reached out for comment, Pfizer Canada’s media relations team could not provide further details about the delay or the parties responsible.
This is not the first time Pfizer’s EpiPen monopoly has come under fire. One of Pfizer’s companies, Meridian Medical Technologies (MMT), received a warning letter from the United States’ Food and Drug Administration (FDA) in September 2017. The letter outlined MMT’s poor quality control and its failure to thoroughly investigate customer complaints and product defects.
“Your […] data shows that you received hundreds of complaints that your EpiPen products failed to operate during life-threatening emergencies, including some situations in which patients subsequently died,” the letter from the FDA read.
In the same correspondence with the Tribune, Pfizer Canada admitted that it had seen “some impact on manufacturing capacity” as a result of the FDA warning.
A shortage of EpiPens doesn’t just mean a manufacturing hassle for Pfizer; this crisis has extreme consequences for many people. A mother in rural Ontario, for example, was reluctant to use her son’s EpiPen during her own severe allergic reaction for fear that it would leave her son—who also suffers from life-threatening allergies—vulnerable during the shortage.
According to a country-wide study conducted by allergy and immunology specialist Moshe Ben-Shoshan and his colleagues at the McGill University Health Centre (MUHC), less than half of patients actually use their EpiPens during anaphylaxis before arriving at the emergency room, often because they are unaware of the severity of their reaction. Even more surprisingly, these statistics were taken before the shortage was announced.
In response to the crisis, Health Canada made an announcement that those who are vulnerable should keep their old EpiPens if they can’t get a new one. However, hoarding EpiPens is only a temporary solution, as they have a relatively short shelf-life of approximately 18 months.
“[An] expired EpiPen will also be effective, although less potent,” Ben-Shoshan said to the Tribune.
As well as encouraging the use of expired EpiPens to meet the demand, the Minister of Health has recently signed a temporary agreement to allow the distribution of AUVI-Q, an EpiPen alternative. Produced by the drug company Kaléo, AUVI-Q is another adrenaline auto-injector now sold for around $170 in pharmacies across the country; they are significantly more expensive than the typical $100 price of an EpiPen.
Moreover, the eggs-in-one-basket model isn’t doing Canada any favours.
“Having different types of manufactures for an epinephrine auto-injector will help reduce the risk of such shortage,” Ben-Shoshan said.
EAIs aren’t the only drugs in low supply. Drugshortagescanada.ca, a third-party website under contract with Health Canada, provides daily updates on drug shortages and discontinuations. According to a document published on the website, drug shortages in Canada have become increasingly common in the last decade.
Drug manufacturers have both a legal and a public responsibility to prevent drug shortages, which means ensuring adequate quality assurance, risk management, and contracting back-up suppliers to guarantee the entire drug supply chain runs smoothly.