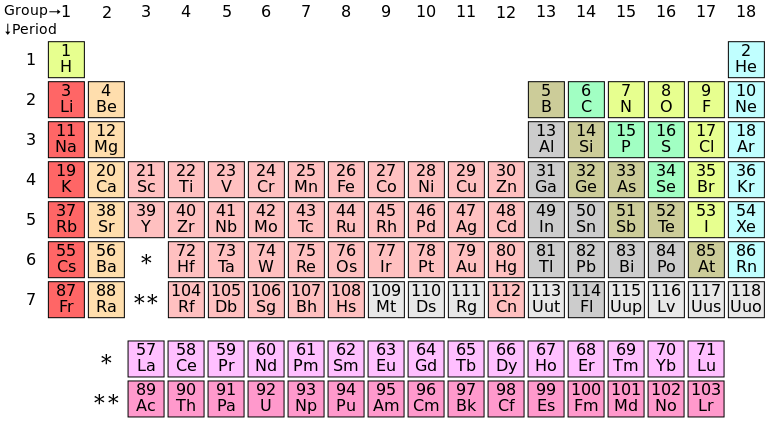
The universe is expanding—and so is the periodic table. Four new elements have recently been added to complete the seventh row. Though the elements were discovered over the last year, it’s only now that the International Union of Pure and Applied Chemistry (IUPAC) has released the element names for public review. If you were thinking of naming one after your dog, though, think again. IUPAC has strict guidelines: New elements are exclusively named after mythology, minerals, places, properties, or scientists.
Element 113 will be nihonium (Nh), for “Nihon”, the Japanese word for Japan. The patriotic name has special significance because nihonium is the first element to be discovered in Asia. The researchers at the RIKEN Nishina Center for Accelerator-Based Science hope “that pride and faith in science will displace the lost trust of those who suffered from the 2011 Fukushima nuclear disaster.”
Meanwhile, elements 115 and 117 also honour geographical regions. There’s moscovium (Mc), for Moscow, and tennessine (Ts), for Tennessee. Moscovium and tennessine follow a long tradition of naming elements after locations significant to their discovery, like germanium named after Germany or scandium named after Scandavania.
Finally, oganesson (Og) is the proposed name for element 118, after Russian chemistry professor Yuri Oganessian. Born in 1933, Oganessian is credited with discovering some of the heaviest elements on the periodic table, known as transactinoid elements. This would be the second time an element has been named for a living person, after chemist Glenn Seaborg was recognized with seaborgium, element 106. Meanwhile, element 104, rutherfordium, honours McGill’s own physicist, Ernest Rutherford.
The new elements have actually been on the periodic table for a while, hiding in plain sight under the unremarkable placeholder names of ununtrium, ununpentium, ununseptium, and ununoctium. However, it’s only now that the discovery teams have been invited to propose the new names.
Dr. Jan Reedijk, who coordinated the efforts between IUPAC and the research teams said, “I see it as thrilling to recognize that […] these new names also make the discoveries somewhat tangible.”
Tangible, however, is not how these elements could be described. They’re superheavy, which means that each element’s nucleus has a huge number of protons. To create a new superheavy element, researchers bombard heavy elements with slightly lighter elements to artificially form, if only for a moment, a novel chemical matter. With a super short half life, the transactinoid elements only exist briefly in the laboratory before decaying.
“A particular difficulty in establishing these new elements is that they decay into hitherto unknown isotopes of slightly lighter elements that also need to be unequivocally identified,” said professor Paul J. Karol of IUPAC.
As the hurdles for discoveries get higher, elements are manufactured in increasingly impossible conditions, which begs the question—how big will the periodic table get? That depends on how many protons can physically fit into an atom’s nucleus. Predictions range widely, from a maximum of 137 to 184 protons. One thing’s for sure—the periodic table’s seventh row may be filled in, but the future likely holds even more additions.