Recent advances in molecular biology techniques are bringing new insights into complex diseases. These insights extend to spinocerebellar ataxias (SCAs), a group of progressive neurodegenerative disorders characterized by the deterioration of the cerebellum—a brain structure critical for balance and movement coordination.
In 2019, between 30 and 48 per cent of SCA patients remained without a genetic diagnosis—a test that identifies the genes responsible for a given disease. This was partly due to the limitations of short-read genetic sequencing, a technique which involves breaking DNA into small fragments before determining the order of DNA units that make up the fragments. While this technique is useful, it struggles to accurately resolve repetitive DNA regions in the genome—a challenge in diagnosing certain types of SCA.
Recognizing the need for improved diagnostic tools, Bernard Brais, the director of McGill’s Rare Neurological Disease Research Group, and his collaborators published a review article analyzing how the advent of long-read sequencing has revolutionized SCA research in recent years.
Unlike short-read sequencing, long-read sequencing can accurately detect repetitive sequences in the genome, revealing previously hidden genetic variants. This technology has led to the identification of three new SCA variants: SCA4, SCA51, and, in particular, SCA27B.
SCA27B stands out from other SCA variants due to its distinct genetic signature: A high number of trinucleotide [guanine-adenine-adenine (GAA)] repeats within an intron—a segment of DNA that does not code for proteins—of the fibroblast growth factor 14 (FGF14) gene.
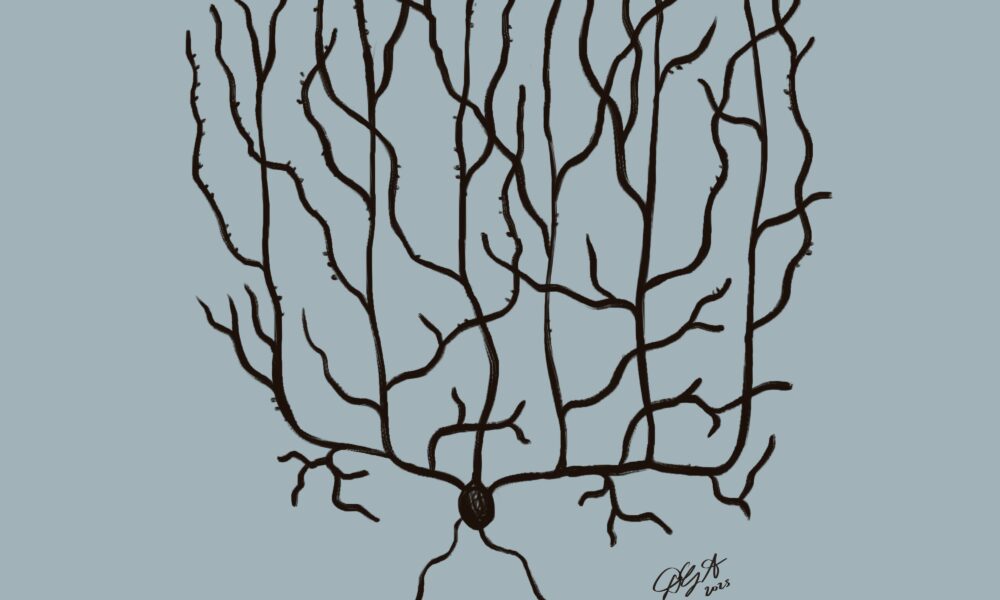
FGF14 plays a key role in stabilizing ion channels crucial for the function of Purkinje cells, specialized neurons in the cerebellum involved in motor control and learning
“We suspect that if [individuals] have less of it, the channels cannot perform as well, and then it leads to all types of problems in the cell,” Brais said in an interview with The Tribune.
Patients with SCA27B typically carry more than 250 GAA repeats in one copy of the FGF14 gene. However, some individuals with fewer than 300 GAA repeats never develop SCA symptoms, suggesting additional genetic considerations influence disease onset.
The purity of the GAA repeat sequence also plays a crucial role in SCA27B development. Asian populations, for example, rarely develop SCA27B since their FGF14 gene contains a mix of triplet nucleotides, making the repeat sequence more stable.
However, individuals inheriting a pure GAA repeat sequence may experience expansion over time, particularly in the cerebellum.
“We think that, with time, the error grows, and it will grow because it is a pure sequence that, for whatever reason, is prone to errors,” Brais commented. “When the DNA is patched up, it makes errors, and [the error] continues to grow.”
Additionally, between 15 and 50 per cent of SCA patients have no family history of this disease. Research suggests that a short 17-base-pair DNA sequence upstream of the FGF14 seems key in stabilizing the GAA repeat sequence length. Therefore, people without this controlling region are more likely to develop SCA27B.
“Somewhere in the history of Europeans, some people probably lost this controlling region, and that makes this region more unstable, so it changes size,” Brais explained. “If it goes past a certain size, it will continue to increase even more, and if it passes a threshold size, then [the person] will develop the disease.”
The discovery of SCA27B represents a major step in refining SCA diagnosis, especially among individuals of European descent. Initially identified in French Canadians, where it accounts for nearly 60 per cent of SCA cases, SCA27B was later recognized as one of the most prevalent SCA variants worldwide.
“It is really worthwhile studying French Canadian genetics to find the cause of diseases,” Brais said.
Beyond ataxias, studying SCA genetics could offer broader insights into neurological disorders associated with aging.
“I think it’s opening the door to a better understanding of aging in terms of memory or movement, like for Parkinson’s disease. But this is not the same; it’s aging in terms of balance,” Brais said.